Fever-scanner company pulls device off market following FDA warning
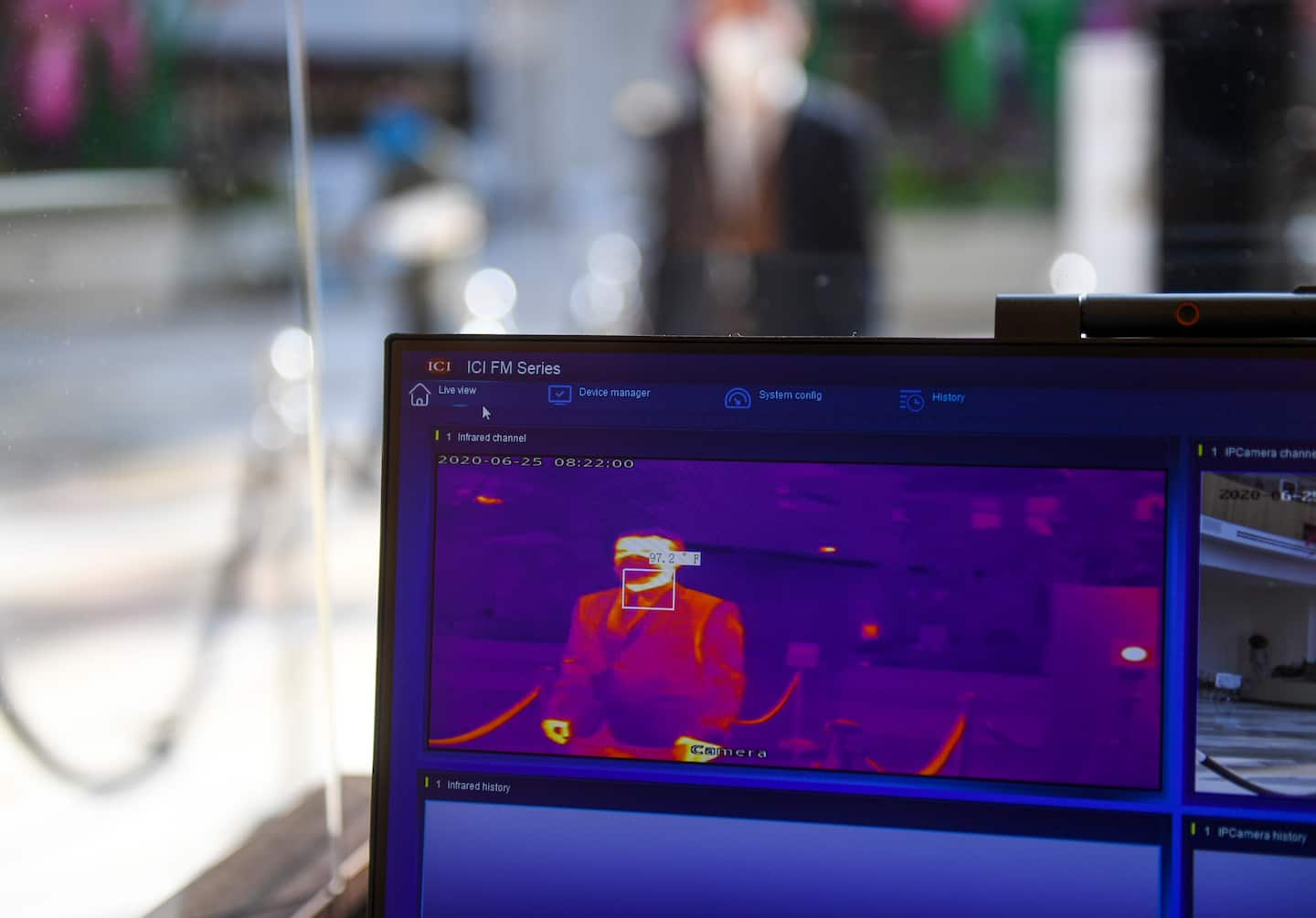
The company, Certify Global, was among seven manufacturers whose products were tested in research first reported Thursday by The Washington Post. The research found critical flaws in thermal-imaging systems’ ability to accurately detect people’s skin temperature. Companies have advertised the systems as a powerful first line of defense in screening people for covid infections.
The FDA on Thursday sent a warning letter to Certify, saying it had not been authorized to sell the device for multi-person scanning, a use the FDA said could lead an infected person to be incorrectly assessed as healthy. The FDA also issued a public alert that similar devices from Certify and other companies could be improperly used in a way that could lead to “potentially serious public health risks.”
Certify spokeswoman Jasmine Neisser said in a statement to The Post that the company is “pulling the InfinityX for the time being as we look into the FDA’s concerns.” She added, “We are quickly pivoting to work on ensuring InfinityX will meet and exceed all their requirements in the future.” In a later email to The Post, she said Certify was “retiring the InfinityX for the time being due to the FDA’s concerns.”
The company had advertised the system as having “advanced AI technology” that can reliably measure temperatures in crowded places at a distance of up to 10 feet. The company also promoted the system for “Multi-Person Scanning,” “Easy Operating” and “Plug & Play Set Up.” Neisser said the 10-feet distance still advertised on Certify’s website is “outdated information.”
Neisser said the company’s SnapXT Pro system is in the process of being FDA approved and is designed to scan one person at a time. The FDA warning letter had said both the InfinityX Pro and the SNAP XT PRO HID had been advertised for scanning multiple people at one time.
Neisser said the company is “in the process of removing any multi-scanning functionalities from the SnapXT Pro HID.” She acknowledged those features are “built into the software” but said they are “not a core part of the product” and have “not been activated for any customers up to this point.”
Based in Maryland, Certify has said in marketing materials that its thermal-scanner systems are now operating in casinos, hotels and other public venues. NFL spokesman Brian McCarthy said Certify scanners are now operating in roughly a dozen of the NFL’s 30 stadiums, but it’s unknown yet which of the company’s scanners are used and whether they are used for single- or multi-person scans. Neisser said the NFL uses SnapXT Pro and SnapXT Pro HID “exclusively for single-scanning purposes.”
“None of the stadiums had InfinityX,” she said, noting “this is a new product and only about 20 have been sold total.”
Certify’s SnapXT system was among the seven thermal-imaging scanners tested by the surveillance research group IPVM, which found that the scanners routinely “normalized” its readings in a way that could overlook elevated skin temperatures during use.